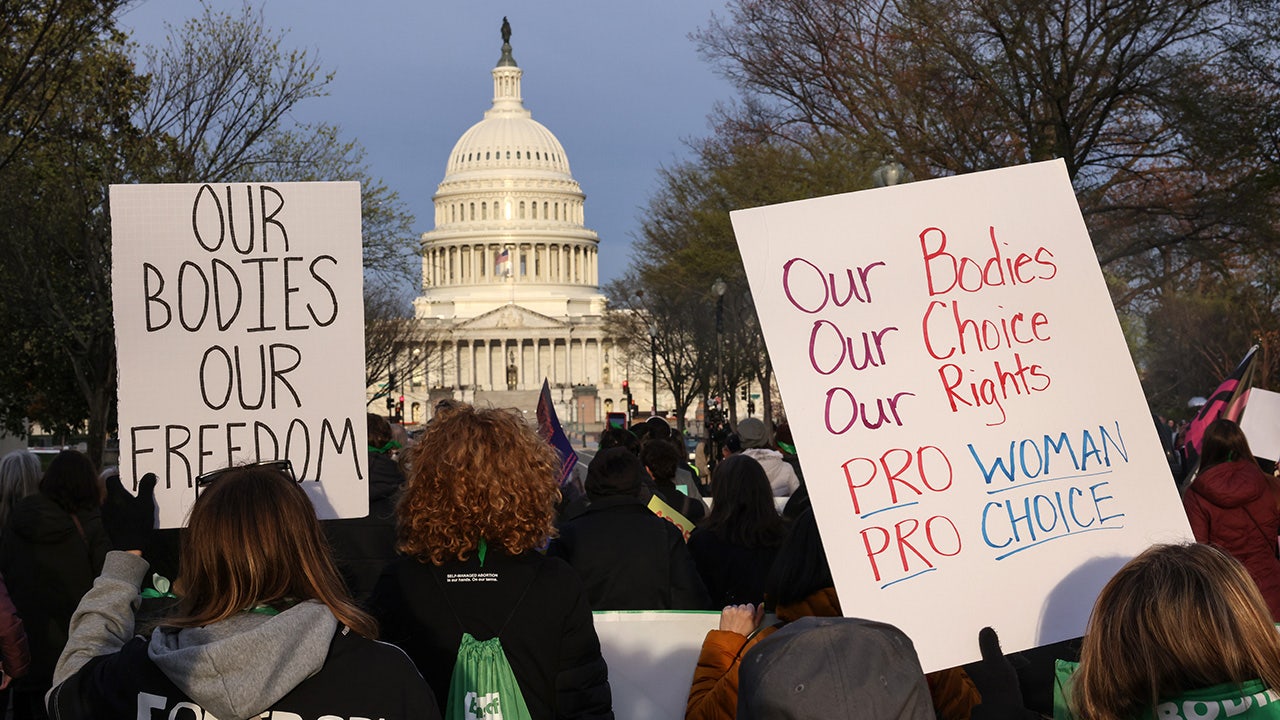[ad_1]
Pro-life leaders are accusing the Food and Drug Administration (FDA) of working with a “reckless disregard for women’s health and safety” over the company’s dealing with of a controversial abortion tablet that is now on the middle of a high-stakes excessive court docket case.
The U.S. Supreme Court on Tuesday heard arguments in a case that would curb nationwide entry to abortion tablets equivalent to mifepristone — the drug that is drawn the ire of the pro-life group, together with March for Life President Jeanne Mancini.
“The FDA’s removal of nearly all safeguards around the dangerous abortion drug mifepristone has needlessly put women and girls at risk for suffering severe — even life-threatening — complications without the ongoing care of a medical provider,” Mancini mentioned. “We hope the FDA will be held accountable for failing to meet its own standards when it comes to abortion drugs. Such reckless disregard for women’s health and safety is unacceptable from an agency tasked with protecting it.”
The case, FDA v. Alliance for Hippocratic Medicine, facilities round mifepristone. Approved by the FDA in 2000, the remedy is used alongside one other drug to finish a being pregnant as early as 10 weeks gestation.
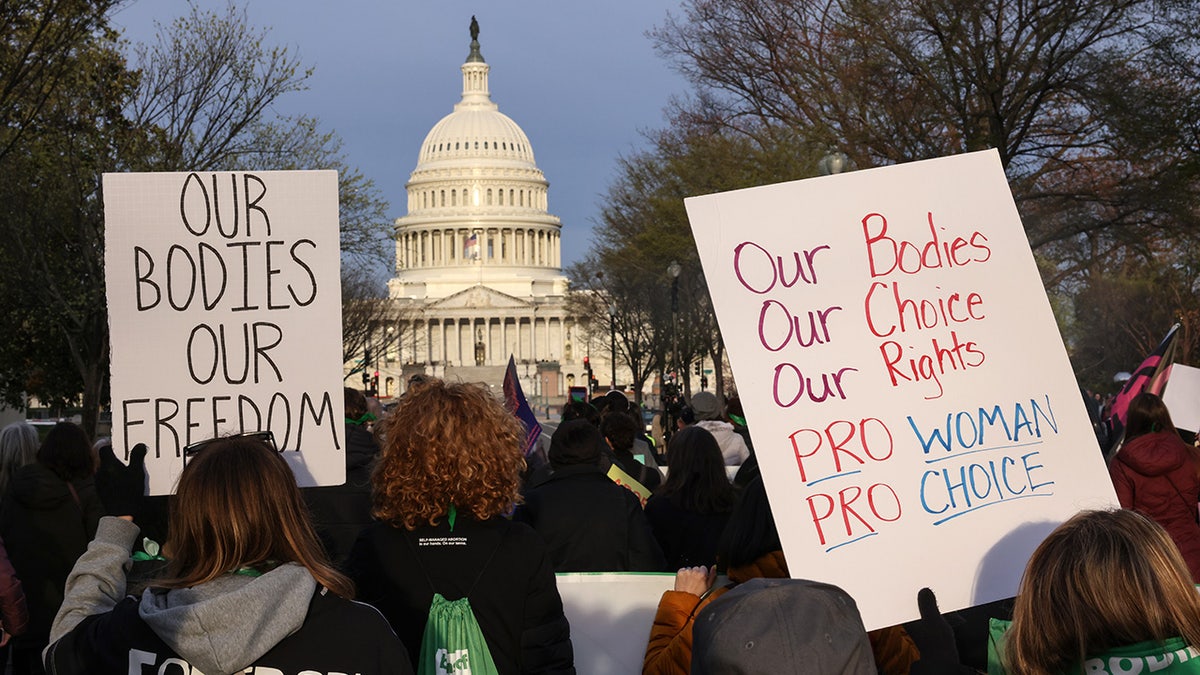
Demonstrations occurred outdoors the U.S. Capitol and Supreme Court on Tuesday amid arguments over mifepristone. (Jemal Countess/Getty Images for Womens March)
The FDA took strides in 2021 — the yr earlier than the Supreme Court’s June 2022 ruling that overturned Roe v. Wade — to make mifepristone extra accessible, together with by eradicating the requirement for sufferers to see a physician in individual to get a prescription.
But months after the Supreme Court’s landmark summer season determination, a federal appeals court docket in Texas dominated the FDA’s coverage change was unlawful, as was an earlier replace that prolonged the tablet’s beneficial utilization by a number of weeks. The excessive court docket is now reviewing that call.
“Bowing to pressure from the abortion industry, the FDA loosened the safety requirements of mifepristone which still has a black box warning,” National Right to Life President Carol Tobias mentioned. “Drugs that come with black box warnings have the most dangerous side effects and safety concerns, yet the FDA is okay with mifepristone being prescribed and even mailed to women without an in-person exam.”
FORMER SUPREME COURT JUSTICE STEPHEN BREYER SOUNDS OFF ON DOBBS DECISION: ‘TOO MANY QUESTIONS’
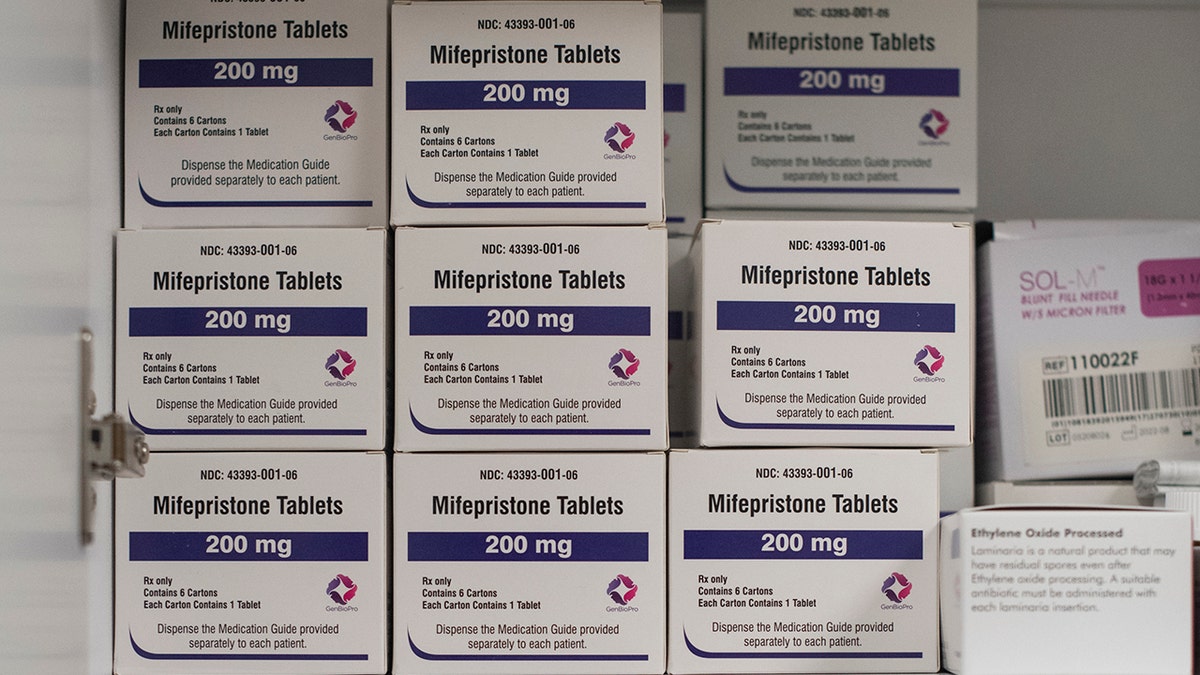
A cupboard containing Mifepristone is seen in Wellspring Health Access clinic in Casper, Wyoming. (Rachel Woolf for The Washington Post by way of Getty Images)
SBA Pro-Life America President Marjorie Dannenfelser informed Fox News Digital that she “was proud to stand with women harmed by abortion drugs courageously sharing their stories, and with the doctors caring for them, in contrast to the abortion industry that leaves women to suffer alone.”
“Today we are standing up to say women’s health matters and the FDA must do its job. We urge the Supreme Court to uphold safeguards for women and girls,” she mentioned.
Erik Baptist, senior counsel of Alliance Defending Freedom, mentioned that pro-life groups are “simply asking the Court to reinstate the original standards that were in effect for over 15 years.”
Meanwhile, pro-choice groups are sustaining that mifepristone is a secure and demanding drug within the post-Roe panorama of reproductive well being.
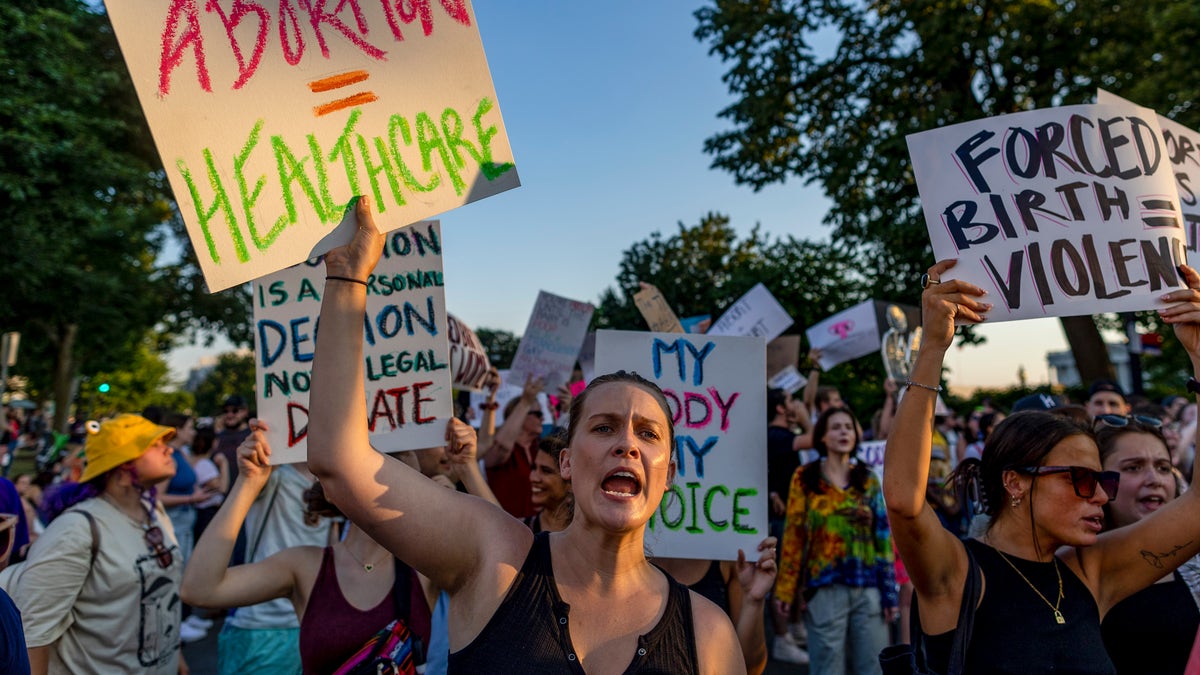
Protesters collect within the wake of the choice overturning Roe v. Wade outdoors the U.S. Supreme Court in Washington, D.C., on June 25, 2022. (Tasos Katopodis/Getty Images)
“Decades of research and clinical experience have demonstrated that mifepristone is extraordinarily safe and effective, including when dispensed by pharmacies or through the mail,” Rabia Muqaddam, senior employees lawyer on the Center for Reproductive Rights, informed Fox News Digital. “There is no legitimate science disputing this research. Instead, the plaintiffs in the case rely on widely discredited and even retracted research articles and the say-so of anti-abortion zealots.”
CLICK HERE TO GET THE FOX NEWS APP
Muqaddam added: “If the Supreme Court sides with the plaintiffs and their junk science over the FDA and decades of high-quality research, countless people will suffer as will the ability of Americans to access important drug innovations.”
Planned Parenthood President Alexis McGill Johnson forged the case as a “threat” to ladies’s rights — and extra.
“Beyond the threat to our fundamental rights, the very existence of this case puts every other FDA-approved medication at risk of being taken off the market or restricted for political reasons,” Johnson mentioned. “That is reason enough to be clear about how serious this case is for the future of sexual and reproductive health care and everything beyond.”
[ad_2]
Source hyperlink