[ad_1]
Food allergy victims have a brand new weapon of their combat towards extreme signs.
The U.S. Food and Drug Administration (FDA) has given the injectable Xolair (omalizumab) the inexperienced mild to be used in lowering the danger of life-threatening reactions to sure foods.
Xolair was permitted for “immunoglobulin E-mediated meals allergy in sure adults and kids 1 yr or older,” the FDA introduced on Feb. 16.
This is the primary treatment the FDA has permitted to scale back allergic reactions after unintended publicity to a number of varieties of meals, the company said.
GLUTEN-FREE LIFESTYLE: DEBUNKING MYTHS AND DECIDING IF THE DIET IS RIGHT FOR YOU
Robert A. Wood, M.D., was the principal investigator of the multicenter research that led to the FDA approval.
“Treatment options, aside from strict avoidance, have been very limited for the millions of Americans with severe food allergies,” Wood, director of the Division of Pediatric Allergy, Immunology and Rheumatology at Johns Hopkins University School of Medicine in Baltimore, Maryland, advised Fox News Digital.

The FDA has given the injectable Xolair (omalizumab) the inexperienced mild to be used in lowering the danger of life-threatening reactions to sure foods. (iStock)
“The lives of these patients and their families are often consumed by fear of accidental exposure to food allergens — and even with strict avoidance, accidental exposures are common.”
“The approval of Xolair for the treatment of food allergy will be very meaningful, and potentially even life-changing, for people with food allergies,” Wood added.
HERE’S WHY YOU’RE BLOATED — AND WHAT TO DO ABOUT THE COMMON DIGESTIVE CONDITION
Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA), a nonprofit headquartered in Maryland, was not concerned within the treatment analysis however spoke with Fox News Digital in regards to the current approval.
“The stress of living with food allergies can weigh heavily on people and their families, particularly when navigating events like children’s birthday parties, school lunches and holiday dinners with friends and family,” Mendez stated.
“Given the growing prevalence of food allergies, this news offers hope to the many children and adults who may benefit from a new way to help manage their food allergies.”

Milk, eggs, fish, crustacean shellfish, wheat, soy, peanuts, and tree nuts account for probably the most severe allergic reactions within the U.S. (iStock)
Individuals should nonetheless keep away from foods they’re allergic to, even when they take Xolair, the FDA famous within the announcement.
“This newly approved use for Xolair will provide a treatment option to reduce the risk of harmful allergic reactions among certain patients with IgE-mediated food allergies,” Kelly Stone, M.D., PhD, affiliate director of the Division of Pulmonology, Allergy and Critical Care within the FDA’s Center for Drug Evaluation and Research, stated in an FDA information launch.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs.”
Risk reducer, not treatment
Xolair, made by Genentech in California, just isn’t permitted for the speedy emergency remedy of allergic reactions. It can be not an alternative to present emergency remedies, the federal company said.
Such emergency remedies embrace doses of epinephrine and EpiPens to forestall anaphylaxis, which is a extreme allergic response that may probably be deadly, well being specialists advised Fox News Digital.

Individuals should nonetheless keep away from foods they’re allergic to, even when they take Xolair, the FDA famous within the announcement. (iStock)
Nearly 6% of U.S. adults and kids endure from meals allergy symptoms, in accordance to the Centers for Disease Control and Prevention (CDC) — and greater than 40% of kids with meals allergy symptoms within the U.S. have been handled within the emergency division.
Dr. Fred Davis, affiliate chair of emergency drugs at Northwell Health in New Hyde Park, New York, stated he sees a quantity of allergic reactions from publicity to meals.
“This drug may be able to lower that risk,” he advised Fox News Digital.
“Remember that this is a preventative drug, not a medication to be used after exposure when one is having an acute allergic reaction,” Davis cautioned.
“This news offers hope to the many children and adults who may benefit from a new way to help manage their food allergies.”
“The recent FDA approval of Xolair for food allergies marks another important step forward for the 33 million Americans living with this condition,” Dr. Susan Schuval, chief of the Division of Pediatric Allergy/Immunology at Stony Brook Children’s Hospital on Long Island, New York, advised Fox News Digital.
“Although Xolair is not a cure for food allergies, its use may lessen the risk of severe reactions from accidental food exposures. Patients will still need to practice food avoidance and carry epinephrine injectors,” Schuval stated.
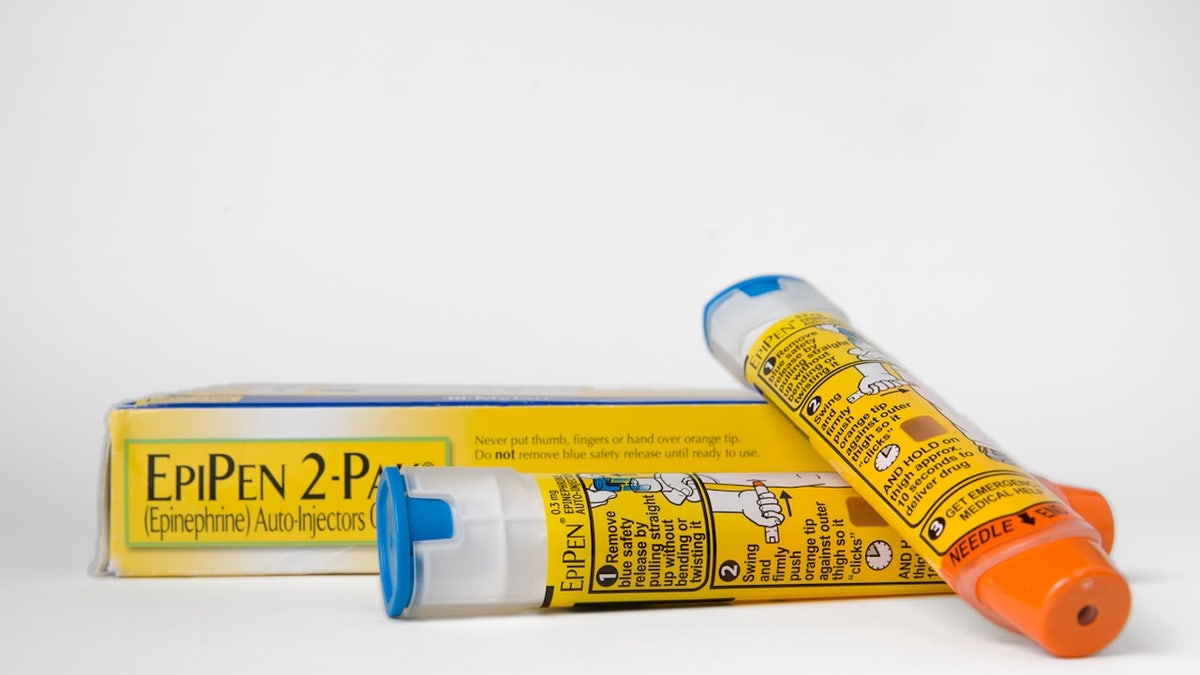
Emergency remedies embrace doses of epinephrine and EpiPens to forestall anaphylaxis, which is a extreme allergic response that may probably be deadly. (iStock)
As there’s at the moment no treatment for meals allergy symptoms, the CDC recommends strict avoidance of any foods that trigger them.
Milk, eggs, fish, crustacean shellfish, wheat, soy, peanuts, and tree nuts account for probably the most severe allergic reactions within the U.S., per the company.
IgE-mediated meals allergy symptoms — probably the most extreme — happen when the physique’s immune system mistakenly perceives a meals particle as a dangerous invader.
AVOID THESE FAD DIETS IN 2024; TRY THESE HEALTHY METHODS OF EATING INSTEAD
A kind of antibody referred to as immunoglobulin E (IgE) contributes to the immune response, which may embrace abdomen points, itching, hives or anaphylaxis, in accordance to a number of well being specialists.
Xolair helps dampen this immune response by focusing on sure receptors within the physique.
“It is an injection that works on blocking IgE, reducing the risk of an allergic reaction, but needs to be taken regularly to work,” Davis advised Fox News Digital.
Research behind the approval
The FDA’s approval determination was based mostly on a research that explored the effectiveness and security of Xolair in 168 contributors starting from infants to adults.
All contributors have been allergic to peanuts and no less than two other foods, which included milk, wheat, egg, walnut, hazelnut or walnut.

A kind of antibody referred to as immunoglobulin E (IgE) contributes to the physique’s immune response, which may embrace abdomen points, itching, hives or anaphylaxis. (iStock)
Participants obtained both Xolair or a placebo for 16 to 20 weeks.
Sixty-eight p.c of those that obtained Xolair have been in a position to tolerate the equal of 2½ peanuts with out a average or extreme allergic response, in contrast to 6% who took the placebo.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Also among the many contributors who obtained the Xolair injections, 67% of folks with egg allergy symptoms, 66% of folks with milk allergy symptoms and 42% of folks with cashew allergy symptoms have been in a position to devour a single dose (1,000 milligrams or higher) of cashew, milk or egg protein with out average to extreme allergic signs.
The company really helpful that a person ought to solely begin the treatment in a well being care setting geared up to handle anaphylaxis.
CLICK HERE TO GET THE FOX NEWS APP
Patients ought to talk about with their well being care supplier whether or not Xolair is the proper selection for them, specialists stated.
Fox News Digital reached out to the FDA for added remark.
For extra Health articles, go to www.foxnews.com/well being.
[ad_2]
Source hyperlink





